Significant figures in physics
Significant figures in physics
Significant figures in physics
History
The concept of significant figures, also known as significant digits or significant digits, is a fundamental concept in scientific and mathematical computation.
The history of significant digits goes back to the development of measurements and the need to express the accuracy and correctness of numerical values. Here is a brief overview of the history of important figures:
Early Development:
The ancient Greeks and Egyptians were among the first to develop methods for measuring and recording numerical values. However, their approach lacked a standardized way to express the accuracy of these measurements.
Introducing decimals:
In the 16th century, the introduction of decimals by mathematicians such as Simon Stevin and John Napier provided an easier way to express and manipulate numbers. This made it possible to increase the accuracy of the calculations, but, of course, did not solve the problem of significant figures.
Scientific Revolution:
In the 17th century, the scientific revolution led to significant advances in experimental methods and the need for accurate measurements. Scientists such as Galileo Galilei and Sir Isaac Newton recognized the importance of precision in measurement, but they had no formalized system for expressing it.
Introducing Uncertainty:
In the 18th century, French mathematician and physicist Pierre-Simon Laplace began developing methods to deal with the uncertainties in measurements. He introduced the concept of error and the idea that every measurement involves uncertainty.
Important figures of the 19th century:
The concept of significant figures as we understand them today began to take shape in the 19th century. William Thomson (Lord Kelvin), an influential physicist and engineer, pioneered the idea of using significant figures to represent the accuracy of measurements. Thomson emphasized the importance of expressing uncertainties and avoiding false precision in scientific calculations.
In 1914, the International Committee on Weights and Measures (CIPM) introduced the International System of Units (SI), which provided a standardized basis for measurements and significant figures. The SI system specifies rules for rounding numbers and determining the number of significant digits in a measurement.
The concept of significant figures remains an integral part of scientific measurement and data analysis. This ensures accurate representation of measurement accuracy and correctness, and enables scientists and engineers to communicate numerical information efficiently.
Standardization and modern use:
In the 20th century, significant figures became more standardized as scientists and mathematicians realized the need for a consistent approach. Organizations such as the Bureau International des Poids et Mesures (BIPM) which translates to the "International Bureau of Weights and Measures" in English,have developed guidelines for expressing measurements and their uncertainties, including the use of significant figures.
Today, significant figures are an integral part of scientific and mathematical calculations. They give an idea of the accuracy of the measurement and help ensure that the calculation results reflect the correct level of uncertainty. Significant numbers are widely used in fields such as physics, chemistry, engineering and many other scientific disciplines.
Defination:
Significant figures (also known as significant figures) are a set of rules used to determine the correct number of digits to include in a measured or calculated value. They help convey the accuracy and correctness of a measurement or calculation.
Rules:
Here are the rules for determining significant figures:
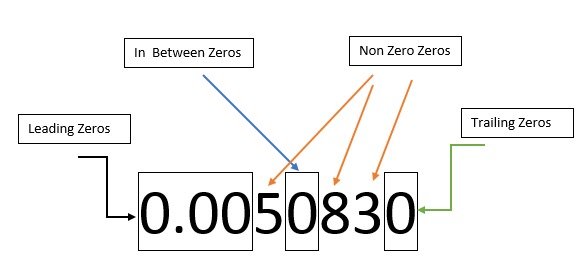
1) Numbers that are not equal to zero are always significant.
For example, in the number 123.45, the non-zero digits are 1, 2, 3, 4, and 5. Each of these digits is a significant digit. As the number 456 has three significant digits.
Here are some other examples:
The non-zero digits 3 and 2 are in the number 0.0032.
In angel number 5001, all four digits (5, 0, 0 and 1) are not zero.
The number 0.007060 has non-zero digits 7 and 6.
Numbers other than zero are important because they indicate a level of accuracy or correctness in a measurement or calculation. When determining the number of significant digits in a value, all non-zero digits are considered significant.
All zeros between non-zero digits are significant. For example, the number 505 has three significant digits.
In the context of significant digits, non-zero digits are all numeric digits from 1 to 9 that are not leading or trailing zeros. These non-zero numbers are considered significant and affect the overall accuracy of the measurement or calculation.
2)Leading zeros (zeros to the left of the first non-zero digit) don't matter.
For example, the number 0.045 has two significant digits.
In the context of significant digits, leading zeros refer to the zeros that appear at the beginning of a number. Leading zeroes are not considered significant and do not affect the number of significant digits in the value.
Significant digits are the digits of a number that affect its accuracy. Include all non-zero digits and all zeros between significant digits. However, leading zeros are not considered significant because they only indicate the position or scale of a number and do not affect its accuracy.
For example:
The number 0.00345 has three significant digits (345).
The number 0.000987 has three significant digits (987).
The number 0.0506 consists of three significant digits (506).
In these examples, leading zeros do not affect the number of significant digits.
3)Trailing zeros (zero to the right of the last non-zero digit) are important if a decimal point is present.
Trailing zeros in significant digits refer to the zeros that appear after the decimal point and are only there to indicate measurement accuracy. These zeros are considered significant and are taken into account when determining the number of significant digits in the value.
For example:
The number "4,500" consists of four significant digits. Zeros after the decimal point are considered significant because they indicate the accuracy of the measurement.
The number "0.020" has two significant digits. Both the zero before the decimal point and the zero after the decimal point are significant.
The number "1200" has two significant digits. The trailing zeros are not considered significant in this case, as they only serve as a placeholder for the size of the number.
It is important to note that trailing zeros without a decimal point can be ambiguous in terms of meaning. To remove this ambiguity, you can use exponential notation, or you can explicitly specify the number of significant digits using additional notation or context.
For example, the number 12.00 has four significant digits.
Trailing zeros that are just placeholders (no decimal point) are irrelevant. For example, the number 300 has only one significant digit.
For addition or subtraction calculations, the result must be rounded to the same number of decimal places as the least accurate value. For example, if you add 2.35 and 1.9, the result must be rounded to two decimal places because 1.9 is the least accurate value.
For calculations involving multiplication or division, the result should be rounded to the same number of significant figures as the least accurate value. For example, if you multiply 3.2 by 4.567, the result must be rounded to three significant digits because 3.2 is the least accurate value.
These rules help maintain an adequate level of accuracy and ensure that the result of the calculation reflects the uncertainty of the measured values.
Types:
Significant Trailing Zeros:
Significant trailing zeros are the zeros that appear after the decimal point in the decimal representation of the number. These trailing zeros are considered significant because they affect the value and precision of the number. For example:
The number 2,500 has a significant zero at the end, indicating that the value is known to tenths.
The number 0.5000 has three significant zeroes at the end, which indicates that the value is known to ten-thousandths.
Insignificant trailing zeros:
Trailing zeros are zeros that appear at the end of an integer or decimal point without changing its value. These trailing zeros do not affect the precision or size of the number, but can be used for formatting or clarity. For example:
The number 1000 has two meaningless zeros at the end, indicating that the value is known within one.
The number 5.0 has a meaningless zero at the end, indicating that the value is known to the nearest integer.
The meaning of trailing zeros depends on the context and purpose of the number. In some cases, trailing zeros may be removed or treated differently depending on formatting conventions or mathematical operations performed.
Where we use significant figures?
The significant figures are used in scientific and mathematical calculations, as well as in the measurement and analysis of the data. Here are some specific areas where significant figures are commonly used:
Measurements: Measurements use the significant figures to express the precision and uncertainty associated with the quantity being measured. For example, if length is measured with a ruler marked in centimeters, the number of significant digits of measurement will depend on the precision of the ruler.
Calculations: Significant figures are fundamental when performing mathematical calculations using measured values. The result of the calculation should be presented with the same number of significant digits of the less accurate value used in the calculation. This ensures that the result is not presented with greater precision than warranted by the original data.
Scientific relationships: When scientific data is presented, it is important to report measurements and calculations with an appropriate number of significant figures. This helps convey the level of uncertainty and provides an accurate representation of the data.
Scientific Notation: Significant digits are used to express very large or very small numbers in scientific notation. For example, the number 0.00045 would be expressed as 4.5 x 10^-4 (two significant digits).
Data analysis: Significant figures are used in the analysis of data to indicate the level of uncertainty associated with a measurement or calculation. In scientific research, it is important to report the level of uncertainty associated with the results to ensure their reliability and reproducibility.
Reporting and Communication: When numerical values are presented in research papers, reports or other forms of communication, significant figures are used to carefully represent the data and convey a level of accuracy. This allows other researchers or readers to understand the limits and uncertainties associated with the reported values.
Chemistry: In chemical calculations, the significant figures play a fundamental role in determining the accuracy of the experimental results and the accuracy of calculations using the amount of substances. They are used in stechiometry, molecular weight calculations, dilution calculations and other related concepts.
Physics: Significant digits are used in physics to express the accuracy and correctness of measured values such as time, distance, mass, and speed. They are important in calculations involving quantities such as strength, energy, amount of motorcycles and other physical properties.
Engineering: Significant numbers are used in engineering fields such as civil engineering, electrical engineering, and mechanical engineering to ensure the accuracy of calculations, measurements, and design specifications.
In general, significant figures are used in various scientific and mathematical disciplines to provide an accurate representation of measurement, calculation, and data analysis. They help convey the level of precision and uncertainty associated with numerical values and are fundamental to maintaining scientific integrity.
What's Your Reaction?





















