Daniell cell
Daniell cell
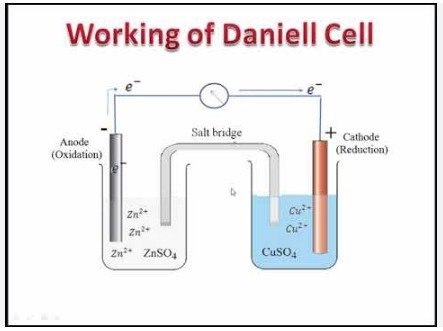
Daniell cell
What is daniell cell?
A Daniell cell is a mechanism that converts chemical energy released by redox reactions into electrical energy.Its electrical potential is 1.1 V. The two separate metals utilised in a Daniell Cell are Zinc (Zn), which functions as the anode, and Copper (Cu), which functions as the cathode. These electrodes are still submerged in ion-based chemical solutions.
Who invented daniell cell?
John Frederic Daniell, a British chemist and meteorologist, created the Daniell cell. In 1836, while employed at King's College in London, he created the cell. Daniell set out to enhance the then-current voltaic cells, which had problems with polarisation and uneven performance. During the 19th century, his creation, known as the Daniell cell, was widely used in numerous electrical applications because it offered notable stability and reliability advantages.
Types:
Two types of cells
Electrochemical cell:
An electrochemical cell can produce an electric current through a chemical reaction or use electricity to generate it. Galvanic, also called galvanic, and electrolytic cells are two different types of electrochemical cells. While electrolytic cells involve non-spontaneous reactions and therefore require an external source of electrons such as a DC battery or AC power supply, electrolytic cells derive their energy from spontaneous redox reactions.
Electrolytic cells:
Any device that converts electrical energy into chemical energy or vice versa is an electrolytic cell. Such a cell usually consists of two electrodes, which can be metal or electronic conductors, separated from each other and in contact with an electrolyte (see), which is usually a dissolved or molten ionic compound.
Difference Between Electrochemical Cells and Electrolytic Cells
1) Galvanic cells convert chemical energy into electrical energy, while electrolytic cells do the opposite.
2) Galvanic cells perform spontaneous chemical reactions while electrolytic cells perform non-spontaneous chemical reactions.
3) An external voltage source must be present in an electrolytic cell to cause a chemical reaction, but not in a galvanic cell (also called galvanic cell).
4) In a galvanic cell, oxidation takes place at the anode and in an electrolytic cell at the cathode.
5) In an electrolytic cell the reduction takes place at the anode and in a galvanic cell at the cathode.
Daniell cell in physics:
In physics, the Daniell cell is often used as an example in the study of electrochemistry and the principles of electrical circuits. Helps illustrate concepts such as electrochemical cells, redox reactions, and electric current.
Daniel's cell is based on the principle of converting chemical energy into electrical energy through a redox reaction. This process involves the transfer of electrons from one electrode (anode) to another electrode (cathode) through an external circuit, creating an electric current.
In Daniel's cell, a redox reaction takes place between zinc and copper. At the anode, the metallic zinc is oxidized, releasing electrons in a half-reaction:
Zn(s) → Zn^2+(aq.) + 2e^-
The zinc ions (Zn^2+) dissolve in the electrolyte (usually sulfuric acid) in the porous tank. At the same time, at the cathode, copper ions of the electrolyte, usually copper sulfate, are reduced to metallic copper, obtaining these electrons:
Cu^2 + (aq.) + 2e^- → Cu (solid.)
Metallic copper is deposited on a copper electrode. This reduction reaction balances the oxidation process taking place at the anode, preventing polarization and allowing the current to be kept constant.
The Daniell cell can be connected to an external circuit, such as a light bulb or voltmeter, to monitor the flow of electrical current and the voltage generated. The direction of current is from the anode (zinc) to the cathode (copper), which is opposite to the flow of electrons.
Studying the Daniell cell allows physicists to investigate several principles, such as the relationship between electrical potential difference (voltage) and current, the concept of resistance in an external circuit, and the chemical reactions that take place inside the cell. It serves as a fundamental example for understanding the electrochemical elements and their applications in physics and other fields of science.
Uses of daniell cell:
The Daniel cell, despite being an older technology, has had several historical and contemporary uses. Here are some notable examples of the use of the Daniel cell:
First source of electricity:
Daniel's cell was one of the first practical and reliable sources of electricity. It played an important role in the development of the first telegraph systems, where it provided the electricity needed for long-distance communication.
Laboratory Research:
The Daniel cell is commonly used in educational institutions and laboratories to demonstrate fundamental concepts of electrochemistry, including redox reactions, half-cell potentials, and the operation of an electrochemical cell.
Standard Voltage Calibration Cell:
The Daniell cell has historically been used as a standard voltage calibration cell. It provides a known and stable output voltage and has served as a reference for measuring and calibrating other electrical devices and systems.
Electroplating:
The Daniell cell, or modifications based on its principles, have been used in electroplating processes. In electroplating, a metal coating is applied to a surface by passing an electric current through a solution containing metal ions. The Daniel cell provides a stable direct current (DC) source for such applications.
Electrochemical Research and Research:
The Daniel cell, with its well-defined and predictable redox reactions, has been used in several electrochemical studies and research projects. It has been used to study factors such as electrode kinetics, corrosion, and the behavior of electrochemical cells.
It is important to note that while the Daniell cell has traditionally served a variety of purposes, it has largely been replaced by more modern and efficient electrochemical technologies such as fuel cells, lithium-ion batteries, and other advanced battery systems for practical applications.
What's Your Reaction?





















