Mass spectrometer, invention, principle, uses
Mass spectrometer, invention, principle, uses
Mass spectrometer, invention, principle, uses
What is spectrometer?
A mass spectrometer is a scientific instrument used to measure the mass and relative abundance of atoms and molecules.
Who invented mass spectrometer?
The mass spectrometer was not invented by one person, but developed over time with the contributions of several scientists. Here are some key figures in the history of mass spectrometry:
JJ Thomson:
At the end of the 19th century, J.J. Thomson, a British physicist, pioneered the study of cathode rays and discovered the electron. His work laid the foundations for the understanding of charged particles and their behavior in electric and magnetic fields, which later became decisive for the development of mass spectrometry.
Francis W. Aston:
Aston, an English chemist and physicist, made significant advances in mass spectrometry in the early 20th century. In 1919 he invented the first practical mass spectrometer, called the "mass spectrograph". Aston used magnetic and electric fields to separate and detect ions based on their mass-to-charge ratio. His work on isotopes earned him the 1922 Nobel Prize in Chemistry.
Arthur J. Dempster:
An American physicist, Dempster contributed to the development of mass spectrometry in the 1910s and 1920s. In 1918, he designed and built the first mass spectrometer capable of high-resolution measurements. Dempster's work focused on the precise determination of atomic masses, and his innovations laid the foundation for later advances in mass spectrometry.
Wolfgang Pohl and John C. Fenn:
In the 1980s, Wolfgang Pohl and John Fenn made important contributions to mass spectrometry, leading to the development of two important ionization techniques.
Paul developed the quadrupole ion trap, a type of mass analyzer that revolutionized the field, with high sensitivity and accuracy in mass measurements. For his contributions, Paul was awarded the Nobel Prize in Physics in 1989.
Together with Koichi Tanaka, Fenn developed electrospray ionization (ESI), a soft ionization technique that allows the analysis of large biomolecules such as proteins and peptides. Fenn was awarded the 2002 Nobel Prize in Chemistry for this achievement.
These scientists, along with many others, have made important contributions to the development and advancement of mass spectrometry over the years. Their work has played a decisive role in making mass spectrometry an indispensable analytical method used in various scientific disciplines.
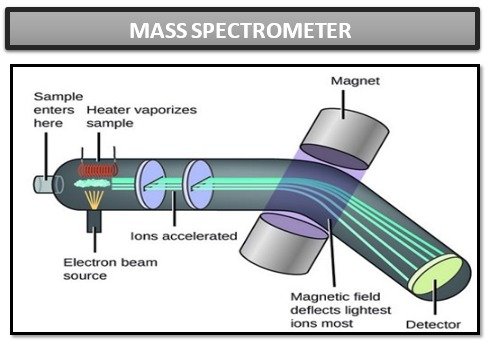
Working principle:
The working principle of a mass spectrometer is based on three main steps: ionization, mass-charge separation, and detection. Here is a detailed explanation of each step:
1)Ionization:
The first step of a mass spectrometer is to convert atoms or molecules in the sample into ions. This is achieved using different ionization methods depending on the nature of the sample. Some commonly used ionization methods are:
- Electron Impact (EI):
In EI, high energy electrons are conducted to the sample, expelling electrons from sample molecules and generating positively charged ions.
- Electrospray ionization (ESI):
ESI is usually used for large, polar molecules. It involves making a fine aerosol from a sample dissolved in a volatile solvent. A high voltage is applied to the solution, which creates positively or negatively charged ions in the gas phase.
- Matrix Laser Desorption/Ionization (MALDI):
MALDI is used for large biomolecules such as proteins and peptides. The sample is mixed with the master compound and applied to a hard surface. Then a laser pulse is applied to cause desorption and ionization of the sample molecules.
2)Mass Charge Separation:
After the sample is ionized, the ions are separated based on their mass-to-charge ratio (m/z) using a mass analyzer. There are several types of mass analyzers commonly used in mass spectrometry:
- Magnetic Sector Analyzer:
This type of analyzer uses a magnetic field to bend the paths of ions based on their mass-to-charge ratio. Ions with different m/z values follow different curved paths, allowing them to be separated.
- Quadrupole Analyzer:
Quadrupole analyzers use a combination of direct current (DC) and radio frequency (RF) electric fields to selectively transfer ions based on their m/z values. By adjusting the applied voltage, some m/z values are allowed to pass through the analyzer while others are filtered out.
- Time of Flight (TOF) Analyzer:
TOF analyzers measure the time it takes for ions to travel a known distance. Ions with higher m/z values take longer to reach the detector than ions with lower m/z values. By measuring the time of flight, the ions can be separated by their mass.
- Ion trap analyzer:
Ion traps use electromagnetic fields to trap ions in a confined space. By controlling the capture potentials, ions with different m/z values can be selectively ejected and detected.
3)Detection:
separated ions are detected in the mass spectrometer detector. The detector generates an electrical signal that is proportional to the number of ions that fall on it. Commonly used detectors include electron multipliers, microchannel plates, and photomultipliers. The electrical signals are then amplified and processed to create a mass spectrum that shows the relative amount of ions as a function of their m/z values.
By analyzing the mass spectrum, scientists can identify the compounds in a sample based on their characteristic mass-to-charge ratio and fragmentation pattern. Mass spectrometry also allows for quantitative analysis by comparing ion signal intensities against known standards or internal standards.
Uses/Application:
Mass spectrometers are widely used in various fields of science. Here are some of the main applications of mass spectrometry:
Identification of chemical compounds:
Mass spectrometry is often used to identify and characterize chemical compounds. By analyzing the mass spectra of unknown compounds and comparing them to databases, scientists can determine the molecular formula and structure of substances.
Quantitative analysis:
Mass spectrometry is used for quantitative analysis, allowing scientists to measure the concentration of certain compounds in a sample. Isotope dilution mass spectrometry (IDMS) is an accurate and precise technique used in fields such as environmental monitoring, clinical diagnostics and drug development.
Proteomics and protein analysis:
Mass spectrometry is a powerful tool in proteomics, the study of proteins and their functions. It provides identification and quantification of proteins in complex biological samples, protein sequencing, analysis of post-translational modifications, and studies of protein-protein interactions.
Metabolomics:
Metabolomics involves the comprehensive analysis of small molecules (metabolites) present in biological samples. Mass spectrometry is used to identify and quantify metabolites, to understand metabolic pathways, to discover biomarkers for diseases and personalized medicine.
Environmental Analysis:
Mass spectrometry is widely used in environmental analysis to detect and measure contaminants, contaminants, and toxic substances in air, water, soil, and biological samples. Help assess environmental impact, monitor compliance, and support environmental remediation efforts.
Forensic Analysis:
Mass spectrometry plays a vital role in forensic analysis where it is used to identify drugs, explosives, toxins and traces. Assists with criminal investigations, toxicology testing and analysis of unknown substances found at crime scenes.
Drug and drug discovery:
Mass spectrometry is an integral part of pharmaceutical product development and analysis. It is used to study drug metabolism, pharmacokinetics, impurity profiling, quality control, and identification of metabolites and degradation products.
Elemental and isotope analysis:
Mass spectrometry allows for accurate analysis of isotopes and elemental composition. It is used in geochronology, nuclear research, archeology and isotope ratio analysis to locate resources and understand natural processes.
Atmospheric and space sciences:
Mass spectrometry is used to study the composition of the atmosphere, aerosols, and trace gases. It helps understand atmospheric processes, monitor air quality, and analyze extraterrestrial samples such as moon rocks and cometary materials.
These are just a few examples of the various applications of mass spectrometry. The versatility and sensitivity of mass spectrometers make them indispensable tools in various scientific disciplines.
What's Your Reaction?





















